A Lecture on Greenhouse Gases: Part 2
Chem 002
Angel C. de Dios
"The Earth system follows laws which scientists strive to understand,"
said Dr. F. Sherwood Rowland, a Nobel laureate in chemistry.
"The public deserves rational decisionmaking based on the best scientific advice about what is likely to happen, not what political entities might wish to happen."
From: Preeminent Scientists Protest Bush Administration's Misuse of Science
Albedo is the percentage of the sunlight that is reflected from the planet - this is dependent on several factors such as cloud cover, color of the planet surface, atmospheric dust, etc. Venus has a very high albedo because of its cloud coverage, which blocks sunlight from reaching the planet's surface.
The Radiative temperature is derived by taking into account the Solar Constant and the Albedo - which gives the amount of solar energy that is absorbed by the planet's surface. This makes the assumption that when materials absorb light, they re-emit some of this energy as heat. Because Venus' cloud coverage reduces the amount of sun's radiation reaching its surface, its predicted surface temperature is colder than that of Earth eventhough Venus lies closer to the sun.
The last column, Surface temperature, demonstrates vividly the Greenhouse Effect. The difference between the Radiative temperature and the actual Surface temperature is the consequence of the Greenhouse Effect.
Venus is HOT! Earth is livable! Mars is COLD!
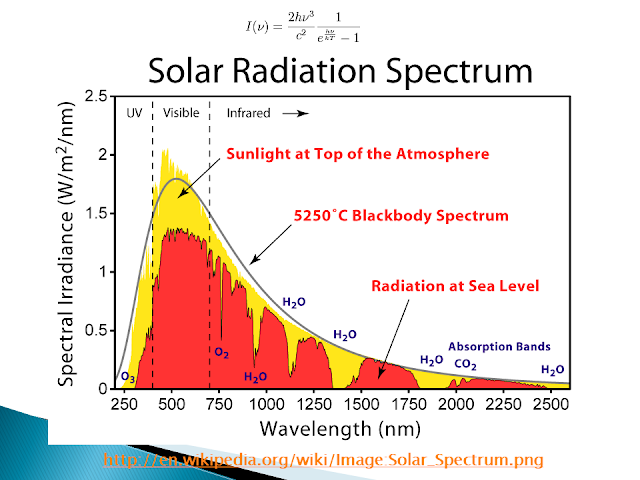
Black-Body Radiation (Max Planck)

Any object at a temperature other than absolute zero emits light. The intensity distribution across the wavelengths is given by Planck's equation above. Hot objects will emit light at shorter wavelengths compared to cooler objects.
The light coming from an object gives us the temperature of that object. The wavelength at the maximum of a radiation curve is inversely proportional to the temperature of the radiating body.
Figure copied from :
http://academic.reed.edu/chemistry/courses/chem101/Handouts/IntrotoGreenhouseEffect.pdf
Original source: "The Chemistry of the Atmospheres", Richard P. Wayne, 1991
Earth's radiation peaks at about 20 micrometers and covers a range of 5 to 100 micrometers. Therefore, most of the radiation absorbed by planet earth will be emitted at these longer wavelengths.
Table copied from
The pressure the atmosphere is related to the surface temperature of the planet.
Venus is HOT! Earth is livable! Mars is COLD!
Venus is 92 atm, Earth is 1 atm, Mars is 0.006 atm
Venus is 92 atm, Earth is 1 atm, Mars is 0.006 atm
Here's a list that will explain exactly what is going on here, by breaking down the categories (the
following percent signs are relative to the original radiation):
100% Incoming solar radiation
6% reflected by atmosphere
20% reflected by clouds
4% reflected by earth's surface
70% absorbed by planet (atmosphere and lithosphere combined)
70% Absorbed by planet (expanding the last category)
16% absorbed by atmosphere
3% absorbed by clouds
51% absorbed by land and oceans
51% Absorbed by surface
23% lost through evaporation
7% lost through direct heating of air
21% of initial radiation to be re-emitted as blackbody
radiation (mostly in infrared)
Molecular rotations and vibrations
Simulations are from http://www.ems.psu.edu/~bannon/moledyn.html
Nitrogen
Oxygen
Molecular vibrations
Water
Carbon dioxide
Ozone
Simulations are from http://www.ems.psu.edu/~bannon/moledyn.html
Nitrogen
Oxygen
Molecular vibrations
Water
Carbon dioxide
Ozone



















Comments
Post a Comment